- The full article entitled “Chapter Open for the Excited-State Intramolecular Thiol Proton Transfer in the Room-Temperature Solution” can be found at the JACS website at https://pubs.acs.org/doi/10.1021/jacs.1c05602
- Authors: Chun-Hsiang Wang, Zong-Ying Liu, Chun-Hao Huang, Chao-Tsen Chen,* Fan-Yi Meng, Yu-Chan Liao, Yi-Hung Liu, Chao-Che Chang, Elise Y. Li,* Pi-Tai Chou*
We report here, for the first time, the experimental observation on the excited-state intramolecular proton transfer (ESIPT) reaction of the thiol proton in solution and solid-state at room temperature. This phenomenon is demonstrated by the derivative of 3-thiolflavone (3TF), namely 2-(4-(diethylamino)phenyl)-3-mercapto-4H-chromen-4-one (3NTF) (see Figure 1), which possesses an -S-H---O= intramolecular H-bond (denoted by the dashed line) and has the lowest lying transition (S0 →S1) at 383 nm. Upon UV excitation, 3NTF exhibits a distinctly red emission maximized at 710 nm in cyclohexane with an anomalously large Stokes shift of 12,230 cm-1 (absorption peak to emission peak). Upon methylation on the thiol group, forming –SCH3 (3MeNTF) that lacks thiol proton, the absorption and weak emission maxima are observed at 370 nm and 472 nm, respectively, with normal Stokes shift. These, in combination with the computational approaches, lead to the conclusion of S-H---O= ® =S---H-O- ESIPT unambiguously. Further time-resolved study renders an unresolvable (<< 180 fs) ESIPT rate for 3NTF, followed by a tautomer emission lifetime, t, of 120 ps. Other thiol analogues including 3TF, 3-mercapto-2-(4-(trifluoromethyl)phenyl)-4H-chromen-4-one (3FTF) were also synthesized. Despite all possessing the S-H intramolecular H-bonds, in sharp contrast to 3NTF, both 3TF and 3FTF are non-emissive. Detailed computational approaches indicate that all studied thiol intramolecular H-bonded compounds undergo thermally favourable ESIPT. However, once forming the proton-transfer tautomer, the low oxidation potential of the sulphur lone-pair electrons brings non-negligible np* contribution to the S1’ state (prime indicates the proton-transfer 0tautomer), for which the relaxation is dominated by the non-radiative deactivation. For 3NTF, the extension of -electron delocalization by the diethylamino electron donating group endows the S1’ state primarily in the pp* configuration, exhibiting the prominent tautomer emission at 710 nm. The results open a new chapter in the field of ESIPT, covering the non-canonical sulphur intramolecular H-bond and its associated ESIPT in solution at ambient temperature.
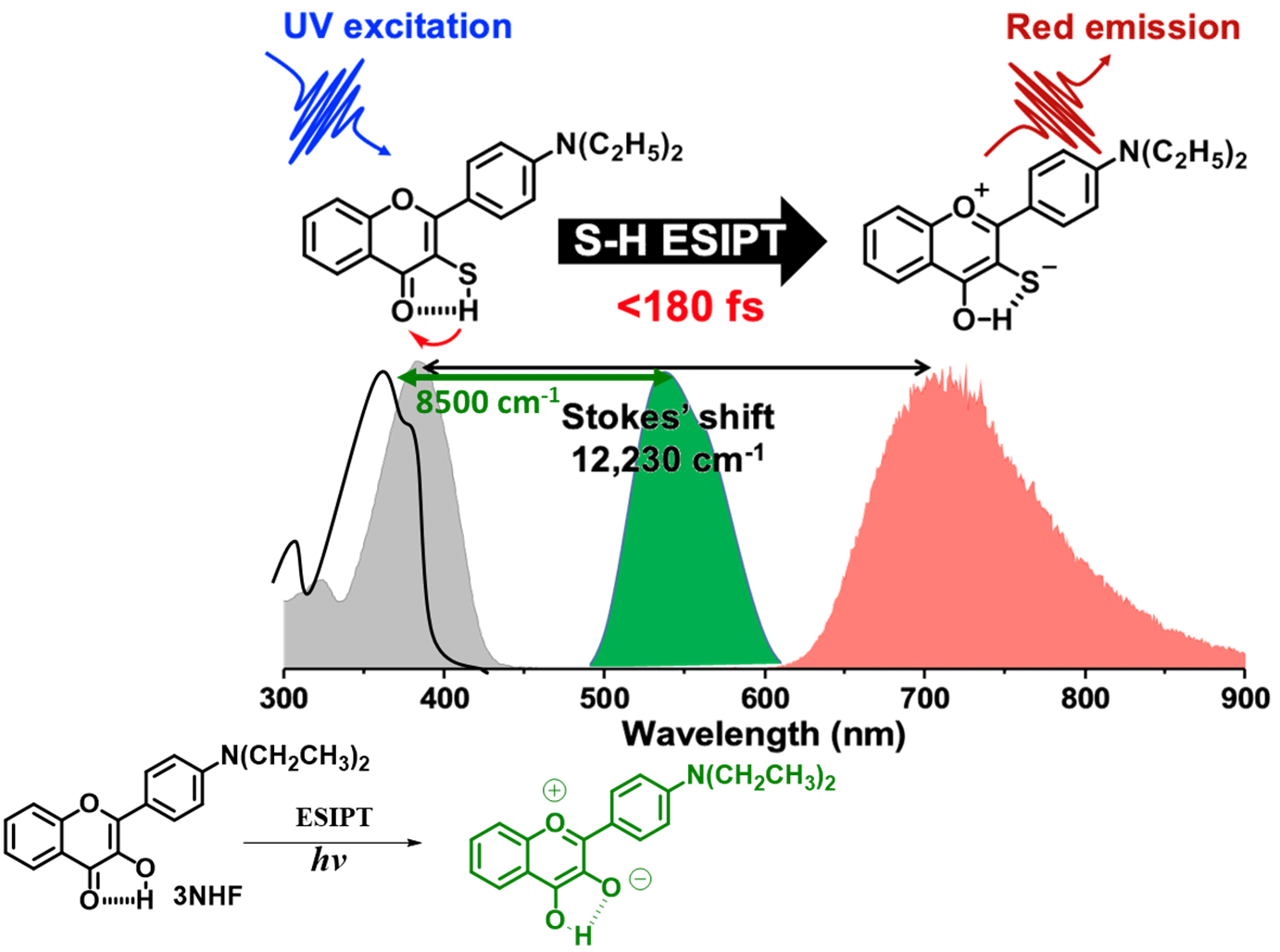
Figure 1. Schematic illustration of S-H excited state proton transfer. Generally, the Stokes shift of the excited state proton transfer of -OH is relatively small, while the Stokes shift of the proton transfer of the excited state proton transfer of S-H is very large. There is a large transparent zone in the middle, which can be used for a wide range of optoelectronic applications, such as mixing other chromophores to achieve a wide color gamut.